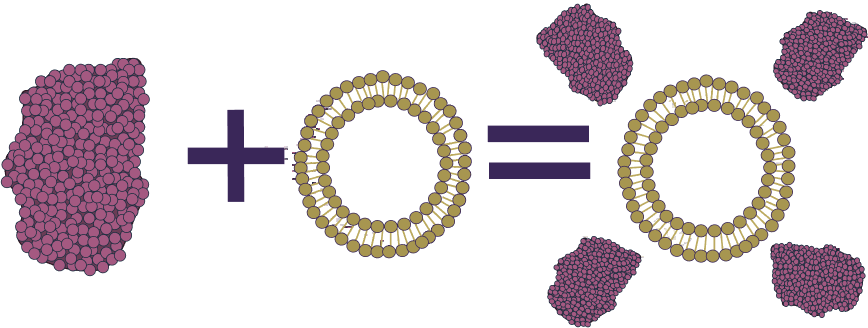
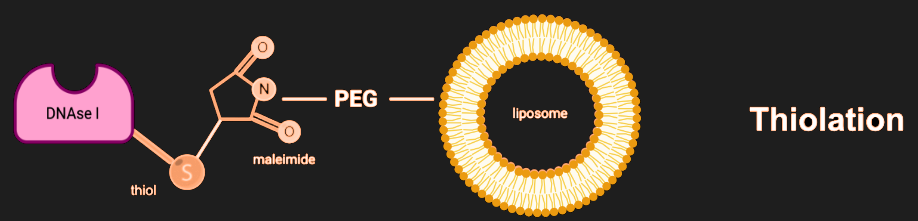
Project Ideas
This project focuses on addressing the challenges posed by bacterial biofilms, structures that play a significant role in infectious diseases and are highly resistant to conventional antibiotic treatments. Follow along to see what factors played a role in our decisions.